Anti-HIV duoCAR-T Cell Therapy Candidate
Our current highest priority cure project is the development of anti-HIV duoCAR-T cell therapy for HIV-1, the virus that causes AIDS if left untreated to replicate in the body and kill CD4+ T cells.
How HIV replicates in T cells
Video Courtesy of Grace Hsu and Janet Iwasa, Animation Lab, University of Utah
Why We Need a Cure
There are currently 38 million people around the world living with HIV, with between 1.7 to 2 million new cases of HIV reported every year.
The current standard of care is antiretroviral drug therapy (ART) which successfully suppresses virus replication and improves the life span of HIV-infected individuals.
However, ART is not a cure for HIV since it only suppresses the virus and thus, requires life-long adherence to drug treatment. Besides treatment failure due to non-adherence, other challenges using ART include poor drug tolerance, development of drug resistance, affordability, and lack of access.
About 47% of the people living with HIV globally are unable to achieve virus suppression due to ART-related toxicities, drug resistance, or lack of access to treatment.
A cure could provide lifesaving treatment for such people and potentially curb the HIV epidemic in places where viral suppression is not achieved.
Even for individuals who are able to achieve durable virus suppression on ART, a cure would significantly improve their quality of life by reducing co-morbidities and making lifelong adherence upon drug therapy unnecessary.
Studies to Date
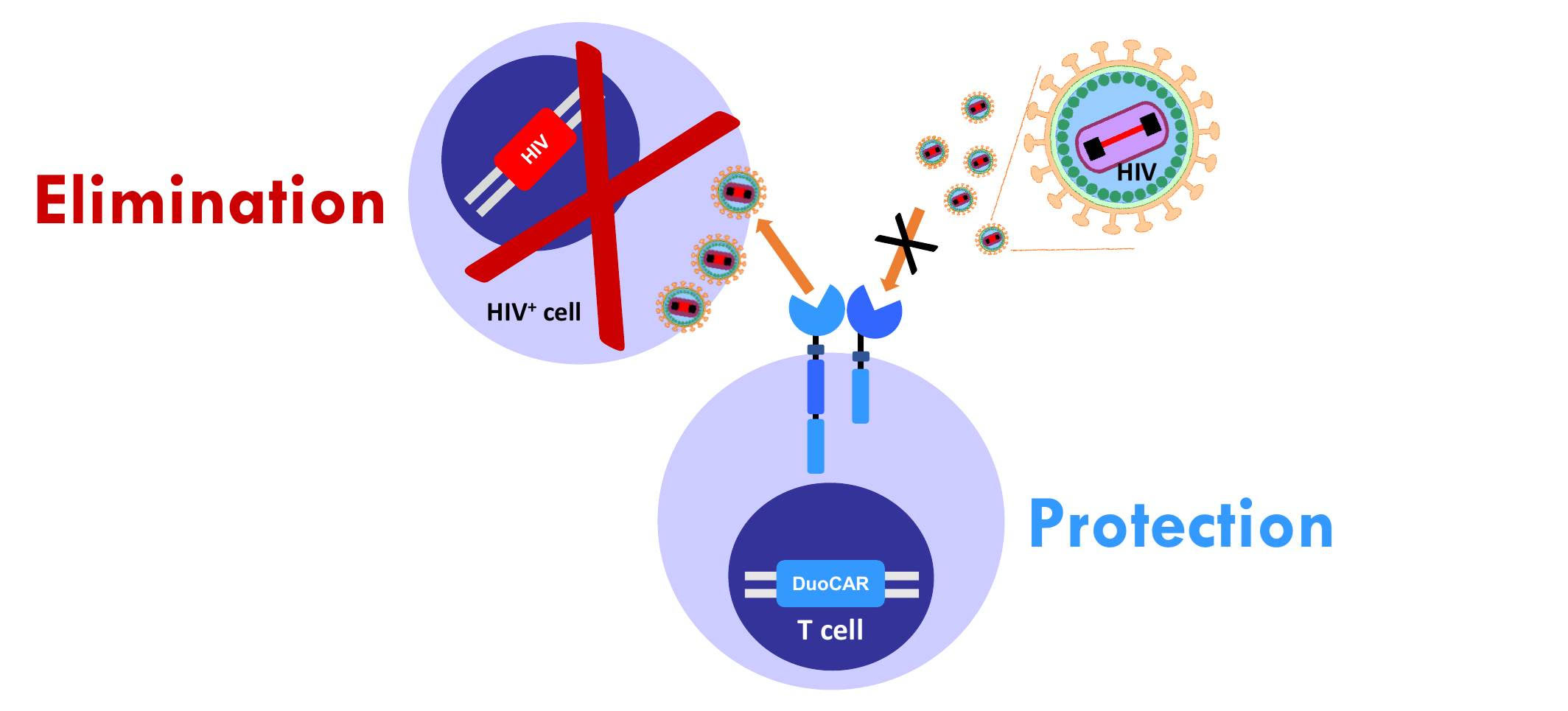
We have developed anti-HIV duoCAR-T cells that have been shown to potently suppress HIV and eliminate actively HIV-infected cells. Our studies with anti-HIV duoCAR-T cells have shown:
- Almost complete inhibition (>96% inhibition) of HIV replication when tested against broad HIV-1 strains found around the world.
- Elimination of actively HIV-infected cells in animal models after infusion of anti-HIV duoCAR-T cells.
- Protection of CD4+ T cells from HIV infection in animal models after a single infusion of anti-HIV duoCAR-T cells. Loss of CD4+ T cells eventually results in AIDS if left untreated.
The above findings are the first-ever to show such effects in an animal model after a single treatment.
Future Studies
A phase I/IIa clinical trial testing anti-HIV duoCAR-T cell therapy has been reviewed by the FDA and has been cleared to begin in 2021 at the University of California San Francisco. Dr. Steven Deeks is the Principal Investigator of the clinical trial. More details of the trial can be found at clinicaltrials.gov with the identifier NCT04648046.
References:
Anthony-Gonda, K., Bardhi, A., Ray, A., Flerin, N., Li, M., Chen, W., Ochsenbauer, C., Kappes,
J., Krueger, W., Worden, A., Schneider, D., Zhu, Z., Orentas, R., Dimitrov, D.S., Goldstein, H.,
Dropulić, B. Multi-specific anti-HIV duoCAR-T cells display broad in vitro antiviral activity and
potent in vivo elimination of HIV-infected cells in a humanized mouse model. Sci. Transl. Med.
2019 Aug; 11: 1-16/eaav5685
https://stm.sciencemag.org/content/11/504/eaav5685
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7136029/

